Guide

The Genu Neurexa is ideal for customers suffering from peripheral nerve damage or paralysis of the leg musculature following a stroke. The Genu Neurexa offers support using the classic three-point system. It prevents hyperextension and facilitates gait training.
Features& Benefits:
Indications:
Hyperextension is prevented using a classic three-point system. Crossed inelastic straps in the hollow of the knee work together with inelastic straps in front as well as lockable lateral joint components. The joint components permit extension and flexion limitation at increments of 10°.PCM (phase change material) serves as the temperature-regulating textile base material.
PCM is very soft and pleasant to wear. It prevents the accumulation of heat so that the orthosis can also be worn for extended periods of time. In order to take full advantage of the material characteristics, the orthosis should be worn directly on the skin. Customers with good hand function can put the orthosis on independently.The Genu Neurexa is machine washable at 40° C.
The product is designed for use on one patient only. Re-use of the product is not permitted.The daily duration of use and period of application are dependent on the medical indication.
Size: XS - XL.
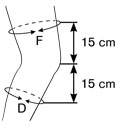
The size selection of the orthosis is based on the knee circumference most important 15 cm below midpatella, second hand 15 cm above midpatella.
Made in Germany.
This product meets the requirements of the 93 / 42 / EEC guidelines for medical devices. This product has been classified as a class I device according to the classification criteria outlined in appendix IX of the guidelines. The declaration of conformity was therefore created by Otto Bock with sole responsibility according to appendix VII of the guidelines.
Others products from Neurexa Line:
| Size | Circ. 15 cm below knee (cm) | Circ. 15 cm above knee (cm) |
|---|---|---|
| XS | 32 – 35 | 38 – 42 |
| S | 35 – 38 | 42 – 46 |
| M | 38 – 41 | 46 – 50 |
| L | 41 – 44 | 50 – 54 |
| XL | 44 – 48 | 54 – 58 |